Mongolia is a landlocked country in East Asia, bordered by Russia to the north and China to the south. The western extremity of Mongolia is only 23 km (14 mi) from Kazakhstan, and this area can resemble a quadripoint when viewed on a map. It covers an area of 1,564,116 square kilometres (603,909 square miles), with a population of just 3.3 million, making it the world’s most sparsely populated sovereign state. Mongolia is the world’s largest landlocked country that does not border a closed sea, and much of its area is covered by grassy steppe, with mountains to the north and west and the Gobi Desert to the south. Ulaanbaatar, the capital and largest city, is home to roughly half of the country’s population.
After the collapse of the Qing dynasty in 1911, Mongolia declared independence, and achieved actual independence from the Republic of China in 1921. Shortly thereafter, the country became a satellite state of the Soviet Union. In 1924, the Mongolian People’s Republic was founded as a socialist state.[12] After the anti-communist revolutions of 1989, Mongolia conducted its own peaceful democratic revolution in early 1990. This led to a multi-party system, a new constitution of 1992, and transition to a market economy.
Approximately 30% of the population is nomadic or semi-nomadic; horse culture remains integral. Buddhism is the majority religion (51.7%), with the nonreligious being the second-largest group (40.6%). Islam is the third-largest religious identification (3.2%), concentrated among ethnic Kazakhs. The vast majority of citizens are ethnic Mongols, with roughly 5% of the population being Kazakhs, Tuvans, and other ethnic minorities, who are especially concentrated in the western regions.
Modern Mongolia inherited a relatively good healthcare system from its socialist period, a world bank report from 2007 notes “despite its low per capita income, Mongolia has relatively strong health indicators; a reflection of the important health gains achieved during the socialist period.” On average Mongolia’s infant mortality rate is less than half of that of similarly economically developed countries, its under-five mortality rate and life expectancy are all better on average than other nations with similar GDP per capita.
Source:Mongolia https://en.wikipedia.org/wiki/Mongolia
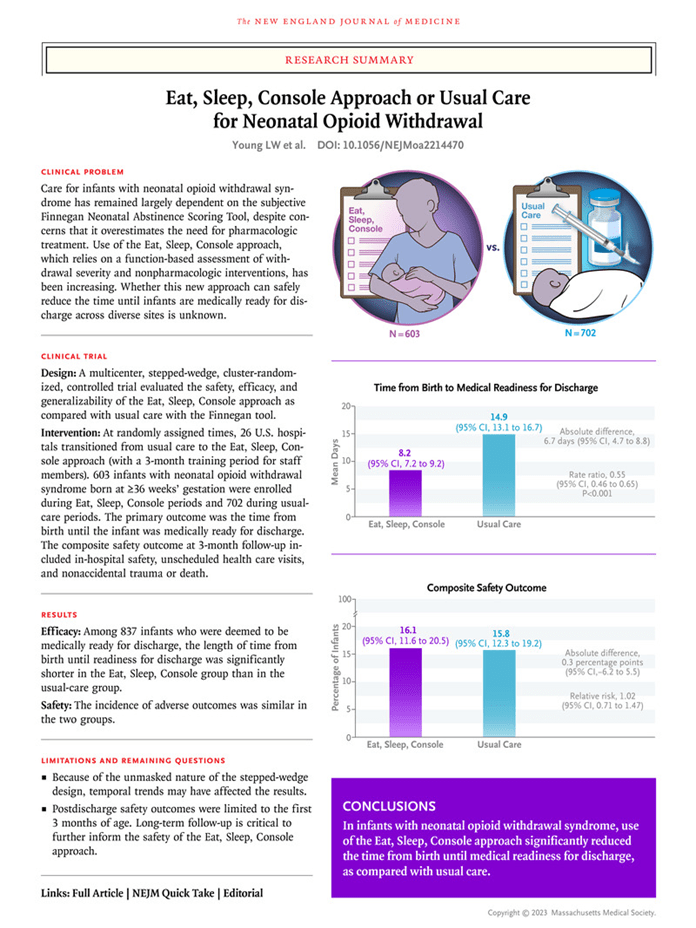
GLOBAL PRETERM BIRTH RATES – Mongolia
Estimated # of preterm births: 14 % (USA 9.56-Global Average: 10.6)
Source:https://data.un.org/Data.aspxd=WHO&f=MEASURE_CODE%3AWHS_PBR

COMMUNITY

How Mongolia Revolutionized Reproductive Health for Nomadic Women
With a series of health reforms and the use of new technologies, Mongolia has dramatically cut its maternal mortality rate and boosted access to maternal healthcare for one of its most vulnerable populations: nomadic women in remote communities.
Published on Jan. 11, 2018 – Written by Didem Tali

After a traumatic home birth in the 1990s, Bayarbat Delgermaa left her nomadic life to move closer to health facilities. But Mongolia’s work to improve maternal healthcare made her confident enough to move back to the Gobi Desert in 2010. Didem Tali
DALANZAGDAD, Mongolia – Bayarbat Delgermaa almost died when she gave birth to her first child in the early 1990s.
“The baby arrived earlier than expected and it was winter time,” Delgermaa, a 47-year-old nomadic herder from Mongolia’s Gobi desert, recalls. She is sitting on the floor of her yurt, which is decorated with woodwork, portraits of horses and the medals that the animals her family breeds have won in local games and festivals.
Delgermaa and her family were living in a remote nomadic community with limited access to medical services when her first child arrived. There was no way to get her to the hospital, so she had to give birth in a yurt, with the help of her relatives. Although she survived the difficult birth and ended up holding a healthy daughter in her arms, the experience traumatized her.
“I wanted to have more children, but didn’t want to go through the same experience,” Delgermaa says. She decided to move her family to the nearest province, where she later gave birth to two more daughters in hospitals with trained medical staff.
Had Delgermaa given birth today, the story of her first delivery might have been a happier one. In the past few decades, Mongolia has made dramatic improvements in maternal health. Through a series of governmental reforms and campaigns, the country has decreased its maternal mortality rate, which tends to be higher in nomadic communities, from 199 deaths for every 100,000 live births in 1990 to 26 in 2015.
Mongolia’s progress on reducing maternal mortality numbers took a hit in 2016, when an economic downturn caused poverty rates to shoot up and led the state to cut budgets for contraceptives and other reproductive health supplies. At the same time, an extreme weather event called a dzud – summer drought followed by severe winter – caused massive livestock loss, exacerbating health issues for women in rural communities. That year, maternal mortality nearly doubled to 48.6 per 100,000 live births.
Still, says Naomi Kitahara, Mongolia representative of the UNFPA, the country has made great strides in reducing the risks for pregnant and birthing mothers, especially with its population so spread out.
“During pregnancy complications, we usually only have two to three hours to save a woman’s life,” says Kitahara. “It’s essential that women across the country have access to the best quality care, especially in life-threatening circumstances.”
The Gobi Desert’s harsh terrain and extreme weather conditions make it difficult for nomadic women to get to clinics to give birth, so Mongolia improved access by opening more health clinics closer to remote communities and tapping into mobile tech. (Didem Tali)
The least densely populated nation-state in the world, Mongolia has seen its urban population rise steadily since the collapse of communism in 1990s. Today, 2 million people out of the country’s total population of 3 million live in urban centers, but a quarter of Mongolians still pursue a traditional nomadic lifestyle.
Many of the health issues in nomadic communities stem from how remote those communities are. In the Gobi Desert and around Mongolia, temperatures can plummet as low as −40C (−40F) in winter and soar to 45C (113F) in summer. Harsh weather conditions and a terrain that is often difficult to navigate pose significant challenges in getting reproductive health services to everyone who needs them.
To address those challenges, Mongolia’s government launched a series of reforms, including increasing the number of maternity waiting homes in all districts, to make them more accessible to nomadic women. Waiting homes are places where women carrying high-risk pregnancies can stay until they give birth, to make sure they can be easily transferred to a nearby medical facility if complications arise before the delivery.
“We’ve managed to reduce the maternal mortality ratio by ensuring delivery in hospitals,” says Naomi Kitahara, Mongolia representative of the UNFPA.
Health authorities also introduced a “two-week” rule, encouraging women in at-risk communities to attend a prenatal clinic two weeks before they are due to deliver, even if they haven’t sought any medical support for their pregnancy before then.
“Now, women from nomadic families come to the provincial or district clinic two weeks before the due date. If there’s a pregnancy-related complication, a skilled birth attendant is on hand to support them,” Kitahara says.
The government has also tapped into the growing mobile and satellite phone networks to help reduce maternal mortality risks. Through a maternal and child health telemedicine network, established in 2008, women across the country can access reproductive health services, including family planning advice and information about cervical cancer.
Two decades ago, few women living in remote communities in Mongolia could access maternal health services. Today, 99.6 percent of births take place at health facilities such as this clinic in the Gobi Desert. (Didem Tali)
The United Nations Population Fund (UNFPA) estimates that around half of deliveries in Mongolia take place in the provinces. Overall, 99.6 percent of births now take place within health facilities that thousands of women didn’t have access to in the 1990s.
Access to maternal health services has improved so much in Mongolia that Delgermaa recently decided it would be safe to return to her nomadic life.
After moving her young family to a province in 1990s to get closer to essential health facilities, she sorely missed being a herder under the endless blue skies of the Gobi Desert. With the new health reforms in place, Delgermaa decided to move her family back out to the desert in 2010, confident that, this time, she and her daughters would be able to get medical help if they need it.
“My daughters will be able to live wherever they want,” Delgermaa says. “Although I am getting older, I don’t need to return to a province myself. I am happy here.”

7 Medical Apps Healthcare Professionals Use
Written By Christine Moore Updated on February 9, 2022

As with other industries, mobile apps have changed the experience of healthcare for both patients and providers. Consumers track exercise, diet, sleep patterns, and even vital signs through their smartphones and watches. Medical practices incorporate digital apps to improve online scheduling, send appointment reminders, and provide telemedicine options. And thanks to a variety of medical mobile apps, physicians now have entire medical libraries at their fingertips to confirm a diagnosis, check drug interactions, and collaborate with other HCPs.
Here are some of the most popular medical apps for doctors, nurses and medical students.
1. Epocrates
iOS and Android — Free version available; Epocrates Plus $174.99/year
Epocrates tops many lists as a must-have medical app for healthcare professionals. Popular features include a robust drug database, including an interaction check for up to 30 drugs at one time, as well as pill identification, ICD-10 code search, and alternative medicine information. The athenaText function connects HCPs to a directory of U.S. physicians and provides direct, secure chat between doctors.
2. Medscape
iOS and Android — Free with registration
This medical reference app is a go-to for current research, clinical information, and continuing medical education (CME). Physicians can earn CME/CE credits through online coursework and track their progress within the app. Medscape can also pull news content covering 34 health fields, as well as information about more than 8,500 drugs, herbals and supplements.
3. PEPID
iOS and Android | Free version available; Specialty suites available for $299.95/year
Designed primarily for emergency room teams, PEPID allows physicians to enter a patient’s symptoms, lab results, and other information to find a likely diagnosis. Providers can browse or search videos of medical procedures, check drug interactions, save favorite content pages, and keep digital notes.
4.UpToDate
Android | Free version available; full subscription starts at $579/year for individual physicians
UptoDate lives up to its name and offers physicians the most current information and answers to clinical questions. The app website touts that more than 1.7 million medical providers use UpToDate. The app also offers CME credits, medical calculators, and built-in email functionality, both with patients and other HCPs.
5. DynaMed Plus
iOS and Android | Free with DynaMed subscription; standalone subscription $399/year for physicians and $99.95 for medical students
This medical reference resource has experts reviewing content multiple times a day to ensure information is current and accurate. DynaMed Plus subscribers can access all DynaMed site tools via mobile, including available electronic health record (EHR) integration.
6. Doximity
iOS and Android — Free for qualifying healthcare professionals
Launched as a networking app for physicians and other clinicians, Doximity may be best known for its Dialer feature, which allows doctors to call patients from their own phones but display an office or hospital number. In May 2020, Doximity launched Dialer Video, providing HIPAA-compliant video connections for doctors practicing telehealth. Physicians can also call patients via Doximity directly from within Haiku, Epic’s mobile electronic medical record app. The Dialer Video feature costs $19.99 per month.
7. 3D4Medical
iOS and Android — Free trial with subscriptions starting at $39.99/year for students and $99.99/year for educators and professionals
When it comes to medical imagery, it’s tough to beat 3D4Medical’s detailed illustrations and interactive 3D models. Particularly helpful for patient education, 3D4Medical offers more than 1,500 videos and animations. The 3D functionality allows physicians to rotate, zoom and change visual perspectives on more than 17,000 anatomical structures.
Source:https://www.healthgrades.com/explore/7-medical-apps-healthcare-professionals-use

5 FREE apps for Medical Students (for productivity, anatomy & focus)
Jan 15, 2022 Daniela C Barragan

Heeey 🙋🏻♀️ Today I’m sharing some of the apps that I use the most at medical school for anatomy, productivity and focus. They’re all free and really simple to use across multiple devices.

Bold – #zozo

Music video by Bold performing “#zozo” off his upcoming 14th album ’90’s Love’. © 2023 440Hz Records, a Division of B Production
Bold Dorjsuren (born on November 16, 1978) is a Mongolian singer, producer, and television personality. He enrolled in “School of music and dance” in 1986 and he graduated as a professional violinist in 1996.


The Global Midwives’ Hub A Resource to Improve Health Outcomes and Advocate for Midwifery
A Resource to Improve Health Outcomes and Advocate for Midwifery Welcome to the Global Midwives’ Hub

The Global Midwives’ Hub is a digital data resource where midwives and midwives’ associations can discover information about the state of their profession and the need for safe delivery services. This information will help them to advocate for a midwife-led continuity of care and to strengthen maternal and newborn health services within their countries and regions.
Developed by the International Confederation of Midwives (ICM) and Direct Relief, the Global Midwives’ Hub is designed with input from midwives, and displays data generated by the midwifery profession as well as official global sources such as the World Health Organization (WHO) and the United Nations Population Fund (UNFPA).
ICM and Direct Relief share a mission to improve maternal and newborn health globally. These two organisations are working together to leverage data to empower midwives in their efforts to inform policy makers of their vital role in saving lives and strengthening communities.
Explore ICM’s Professional Framework
ICM operates according to a Professional Framework that has 10 core elements: a philosophy, essential competencies for midwifery, education, regulation, association, research, a model of practice, leadership, and enabling environment and a commitment to gender equality and JEDI. The elements are deeply woven and entirely interdependent. The ICM SoWMy Survey collected data about 4 elements: association, education, leadership, and regulation.
Learn New Skills
Anyone can use open-source data, free of charge. Learn how to use the Hub to visualise, download, create and share your own data visualisations. Learn New Skills
#MappingMidwives
Midwives’ associations globally are using data analysis to shape the issues and agendas in their countries and to advocate for an improved midwifery profession. Below are a few examples of midwives’ associations who have collaborated and created data products from open data to advocate for improved safe delivery services.
The team behind the Global Midwives’ Hub we will be working with a limited number of additional Midwives’ Associations to create and share midwife focused data products on the Global Midwives’ Hub.
Would you like to register your interest?
Connect with the Team to feature your midwives’ association as #MappingMidwives!
About
The aim of the Global Midwives’ Hub is to increase geographic understanding in the field of midwifery, in order to improve outcomes in Maternal and Newborn Health. The Global Midwives’ Hub is a collaboration between the International Confederation of Midwives and Direct Relief.
- Contact Us
- International Confederation of Midwives
- Koninginnegracht 60, 2514 AE ZH
- The Netherlands
- Phone: +31 (0) 70 3060520
- Email: info@internationalmidwives.org
- https://www.globalmidwiveshub.org/#connect

INNOVATIONS

Probiotics, Prebiotics, Lactoferrin, and Combination Products for Prevention of Mortality and Morbidity in Preterm Infants
A Systematic Review and Network Meta-Analysis – 10/02/2023
Yuting Wang, MD1; Ivan D. Florez, MD, MSc, PhD1,2,3; Rebecca L. Morgan, MPH, PhD1,4; et alFarid Foroutan, PhD1,5; Yaping Chang, PhD1; Holly N. Crandon, MBiotech6; Dena Zeraatkar, MSc, PhD1,7; Malgorzata M. Bala, MD, PhD8; Randi Q. Mao, MD9; Brendan Tao, MD10; Shaneela Shahid, MD, MSc11; Xiaoqin Wang, PhD6; Joseph Beyene, PhD1; Martin Offringa, MD, PhD12; Philip M. Sherman, MD13; Enas El Gouhary, MD11; Gordon H. Guyatt, MD, MSc1; Behnam Sadeghirad, PharmD, MPH, PhD1,6,7

Key Points
Question In premature infants, what association do probiotics, prebiotics, lactoferrin, and their combination have with major morbidity, mortality, and intervention-related adverse effects?
Findings This systematic review and network meta-analysis including 106 trials involving 25 840 preterm infants found that multiple-strain probiotics were associated with reductions in all-cause mortality, necrotizing enterocolitis, feeding intolerance, and hospitalization. When combined with oligosaccharides, multiple-strain probiotics were associated with reductions in NEC and feeding intolerance and the best effectiveness for these outcomes but did not have high-certainty evidence for other outcomes.
Meaning Moderate- to high-certainty evidence shows multiple-strain probiotics alone or possibly in combination with oligosaccharides to be superior to alternative prophylactic interventions.
Abstract
Importance Modulation of intestinal microbiome by administering probiotics, prebiotics, or both may prevent morbidity and mortality in premature infants.
Objective To assess the comparative effectiveness of alternative prophylactic strategies through a network meta-analysis (NMA) of randomized clinical trials.
Data Sources MEDLINE, EMBASE, Science Citation Index Expanded, CINAHL, Scopus, Cochrane CENTRAL, and Google Scholar from inception until May 10, 2023.
Study Selection Eligible trials tested probiotics, prebiotics, lactoferrin, and combination products for prevention of morbidity or mortality in preterm infants.
Data Extraction and Synthesis A frequentist random-effects model was used for the NMA, and the certainty of evidence and inferences regarding relative effectiveness were assessed using the GRADE approach.
Main Outcomes and Measures All-cause mortality, severe necrotizing enterocolitis, culture-proven sepsis, feeding intolerance, time to reach full enteral feeding, and duration of hospitalization.
Results A total of 106 trials involving 25 840 preterm infants were included. Only multiple-strain probiotics were associated with reduced all-cause mortality compared with placebo (risk ratio [RR], 0.69; 95% CI, 0.56 to 0.86; risk difference [RD], −1.7%; 95% CI, −2.4% to −0.8%). Multiple-strain probiotics alone (vs placebo: RR, 0.38; 95% CI, 0.30 to 0.50; RD, −3.7%; 95% CI, −4.1% to −2.9%) or in combination with oligosaccharides (vs placebo: RR, 0.13; 95% CI, 0.05 to 0.37; RD, −5.1%; 95% CI, −5.6% to −3.7%) were among the most effective interventions reducing severe necrotizing enterocolitis. Single-strain probiotics in combination with lactoferrin (vs placebo RR, 0.33; 95% CI, 0.14 to 0.78; RD, −10.7%; 95% CI, −13.7% to −3.5%) were the most effective intervention for reducing sepsis. Multiple-strain probiotics alone (RR, 0.61; 95% CI, 0.46 to 0.80; RD, −10.0%; 95% CI, −13.9% to −5.1%) or in combination with oligosaccharides (RR, 0.45; 95% CI, 0.29 to 0.67; RD, −14.1%; 95% CI, −18.3% to −8.5%) and single-strain probiotics (RR, 0.61; 95% CI, 0.51 to 0.72; RD, −10.0%; 95% CI, −12.6% to −7.2%) proved of best effectiveness in reduction of feeding intolerance vs placebo. Single-strain probiotics (MD, −1.94 days; 95% CI, −2.96 to −0.92) and multistrain probiotics (MD, −2.03 days; 95% CI, −3.04 to −1.02) proved the most effective in reducing the time to reach full enteral feeding compared with placebo. Only single-strain and multistrain probiotics were associated with greater effectiveness compared with placebo in reducing duration of hospitalization (MD, −3.31 days; 95% CI, −5.05 to −1.58; and MD, −2.20 days; 95% CI, −4.08 to −0.31, respectively).
Conclusions and Relevance In this systematic review and NMA, moderate- to high-certainty evidence demonstrated an association between multistrain probiotics and reduction in all-cause mortality; these interventions were also associated with the best effectiveness for other key outcomes. Combination products, including single- and multiple-strain probiotics combined with prebiotics or lactoferrin, were associated with the largest reduction in morbidity and mortality.
Source: https://jamanetwork.com/journals/jamapediatrics/article-abstract/2810095

A prediction model for neonatal necrotizing enterocolitis in preterm and very low birth weight infants
Front. Pediatr., 18 October 2023 Sec. Neonatology Volume 11 – 2023 | https://doi.org/10.3389/fped.2023.1242978 Baoying Feng1,2 Zhihui Zhang3 Qiufen Wei1,2 Yan Mo1,2 Mengmeng Luo4 Lianfang Jing1,2 Yan Li1,2*

Objectives: Neonatal necrotizing enterocolitis (NEC) is a severe gastrointestinal disease that primarily affects preterm and very low birth weight infants, with high morbidity and mortality. We aim to build a reliable prediction model to predict the risk of NEC in preterm and very low birth weight infants.
Methods: We conducted a retrospective analysis of medical data from infants (gestational age <32 weeks, birth weight <1,500 g) admitted to Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region. We collected clinical data, randomly dividing it into an 8:2 ratio for training and testing. Multivariate logistic regression was employed to identify significant predictors for NEC. Principal component analysis was used for dimensionality reduction of numerical variables. The prediction model was constructed through logistic regression, incorporating all relevant variables. Subsequently, we calculated performance evaluation metrics, including Receiver Operating Characteristic (ROC) curves and confusion matrices. Additionally, we conducted model performance comparisons with common machine learning models to establish its superiority.
Results: A total of 292 infants were included, with 20% (n = 58) randomly selected for external validation. Multivariate logistic regression revealed the significance of four predictors for NEC in preterm and very low birth weight infants: temperature (P = 0.003), Apgar score at 5 min (P = 0.004), formula feeding (P = 0.007), and gestational diabetes mellitus (GDM, P = 0.033). The model achieved an accuracy of 82.46% in the test set with an F1 score of 0.90, outperforming other machine learning models (support vector machine, random forest).
Conclusions: Our logistic regression model effectively predicts NEC risk in preterm and very low birth weight infants, as confirmed by external validation. Key predictors include temperature, Apgar score at 5 min, formula feeding, and GDM. This study provides a vital tool for NEC risk assessment in this population, potentially improving early interventions and child survival. However, clinical validation and further research are necessary for practical application.
Source:https://www.frontiersin.org/articles/10.3389/fped.2023.1242978/full
Dr. Ben Courchia, MD | ChatGPT, a revolutionary tool for the modern neonatologist | Delphi 2023

The Incubator Channel Sep 19, 2023
Doctor Benjamin Courchia is a neonatal intensive care physician working in Davie, Florida. He is the director of neonatal innovation at Envision health and HCA University Hospital. He is actively involved in the development and implementation of new technologies to improve the care of critically ill neonates. He is also the director of the chronic lung disease program. He is an adjunct faculty of medicine at Nova Southeastern University. At Delphi 2023, Ben presented how ChatGPT could be used by neonatologists in various contexts, such as research, education and more.
********************************************************

Neonatal ICU mystery: Unraveling the secrets of the prevalent Staphylococcus strain
In a recent study published in Microbial Genomics, researchers investigated the genomes of a group of Staphylococcus capitis isolates from neonates.
Background
NRCS-A, a clone of S. capitis, is prevalent among newborns, a vulnerable population prone to late-onset sepsis. This NAS, a prevalent cause of late-onset sepsis (LOS), lengthens hospital stays, requires invasive procedures, and requires antibiotic treatments, all of which have a severe influence on newborn babies’ long-term health.
Despite a significant incidence of the strain in neonatal intensive care units (NICUs) globally, the mechanisms of NRCS-A are unknown.
About the study
In the present study, researchers analyzed staphylococci isolates obtained from a longitudinal assessment of NAS from gut and skin swabs of NICU-admitted babies.
The study included neonates admitted to neonatal ICUs of Norfolk and Norwich University Hospital (NNUH, United Kingdom) or University Children’s Hospital (Germany) throughout 10-week intervals in 2017 and 2018. The UK unit enrollment occurred between November 2017 and January 2018, whereas the German unit enrollment occurred between January and March 2018.
The researchers examined S. capitis-colonizing neonates admitted to the two NICUs and pathological clinical isolates. Swabs are regularly collected from neonates upon hospitalization and during their stays at both locations for monitoring methicillin-resistant Staphylococcus aureus (MRSA).
Duplicate swab specimens were collected for the current investigation, and staphylococci were isolated. Isolates were obtained from positive cerebrospinal fluid, blood, wound cultures, and urine during the research, and those obtained subsequently were also included.
On admission and every week until discharge, Amie charcoal swabs were used for isolating microorganisms from newborns.
Swabs obtained from the nose, ear, groin, axilla, and stomach were streaked on horse blood agar before incubating at room temperature for 24 hours, and coagulase-negative Staphylococcal organisms were identified following mannitol-salt agar (MSA) sub-cultures, coagulase testing, and matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry.
Clinically relevant S. capitis isolates detected by local departments during routine practice during the investigation period were included, as were further anonymized clinical isolates obtained during regular hematological tests from neonates suspected of having sepsis from the NNUH neonatal ICU in 2018 (seven neonates) and from June to May 2022 (five neonates).
A 15 Staphylococcus capitis-panel was isolated from pre-existing Staphylococcal collections using Amies swabs, and isolates were obtained from adult hematological cultures (in cases of suspected infection) and prosthetic joint infections (PJIs).
Isolates were cultured overnight at 37 °C in Brain Heart Infusion (BHI) broth, and deoxyribonucleic acid (DNA) was isolated, measured, and submitted to polymerase chain reaction (PCR) and whole-genome sequencing.
The pangenome of 138 isolates was evaluated after genome analysis. The phylogeny of Staphylococcus capitis isolates was studied to find traits related to NRCS-A isolates. The National Center for Biotechnology Information (NCBI) protein database was searched for nsr and tarJ gene homologs. Antimicrobial susceptibility tests and pH sensitivity studies were also carried out.
Results
The team discovered 102 S. capitis isolates from four body locations in 159 regular swabs from NICU newborns in the United Kingdom and Germany, 12 from neonates with illness, 11 from blood, and one from skin. The average genome size of all 129 strains was 2.5 Mbp, with 33% GC content.
The team found a three-group population structure: non-NRCS-A strains, NRCS-A strains, and ‘proto-NRCS-A’ strains closely linked to the NRCS-A strains but unrelated to neonatal infections. All bloodstream isolates belonged to the NRCS-A group and were indistinguishable from skin or gut strains.
NRCS-A strains were more resistant to antibiotics and chlorhexidine than other Staphylococcus capitis isolates and could proliferate at higher pH levels. Both the NRCS-A and proto groups had characteristic tarJ and nsr genes. Only NRCS-A isolates exhibited the clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated protein (Cas) system and increased expression genes involved in metal uptake and transport.
The researchers discovered evidence of Staphylococcus capitis NRCS-A transmission in the neonatal ICU, with related strains transferred between newborns and repeated acquisitions by a few neonates. NRCS-A isolates colonized uninfected neonates in the NICU, indicating a possible reservoir for infection.
Researchers discovered genes involved in the higher disease potential of the NRCS-A isolate, including antimicrobial peptide resistance, metal uptake and detoxification, and phage defense.
The genes enabled NRCS-A to persist in the gut, which might explain its success. Multiple antimicrobial resistance (AMR) genes were found in NRCS-A isolates, including fusB (fusidic acid resistance), blaZ (beta-lactamase), mecA (penicillin/methicillin resistance), and AAC(6′)-la-APH(2′)-la (aminoglycoside resistance).
Antiseptic susceptibility differed by geographical location, with S. capitis isolates being more sensitive to octenidine than chlorhexidine. The 50% minimum inhibitory concentration (MIC50) values for octenidine and chlorhexidine were lower in German isolates, whereas they were greater in UK isolates for gentamicin, penicillin, and fusidic acid. No vancomycin resistance was detected; however, roughly a quarter of the patients showed intermediate susceptibility.
Conclusions
Overall, the study findings showed that the most prevalent neonatal strain detected on the skin and gut of uninfected newborns was NRCS-A, which was transmitted and survived in the NICU. The isolate was linked to CRISPR genes and has a full CRISPR-Cas type III-A system.
Carriage isolates were indistinguishable from blood cultures, suggesting that carriage can occur before infection. Strategies to prevent gut colonization may help reduce NRCS infections. The ability to live in the stomach and on the skin aided transmission, and metal uptake and tolerance may be important in NRCS-A biology. Further research is required to devise infection control protocols for NRCS-A.
Journal reference:
Felgate H, Sethi D, Faust K, et al. (2023). Characterisation of neonatal Staphylococcus capitis NRCS-A isolates compared with non NRCS- A Staphylococcus capitis from neonates and adults. Microbial Genomics, 9:001106.

PREEMIE FAMILY PARTNERS

For Preemie Babies, Preschool Plus Parenting Can Spell Academic Success
By HealthDay Sept. 1, 2023 By Cara Murez HealthDay Reporter
Infants born three to six weeks early — considered late preterm — are at risk for learning problems, but they can be overcome, researchers say.
Preschool attendance and sensitive parenting can help them bridge the gap academically, a new study shows.
“Our findings highlight an opportunity for pediatric providers to offer prevention strategies to parents of late preterm infants to mitigate academic risk, and promote academic resilience through sensitive parenting,” said lead author Dr. Prachi Shah, a developmental and behavioral pediatrician at University of Michigan Health C.S. Mott Children’s Hospital in Ann Arbor.
Low level of maternal education, prenatal tobacco use, twins/multiple gestation and male sex increased the risk for deficits in math and reading by kindergarten for late preterm infants, the study found.
Pediatricians can foster sensitive parenting to help these kids, researchers said. They can promote early relational health, where parents provide a safe, stable and nurturing relationship with their children.
“We found that early sensitive parenting experiences were associated with early academic success for late preterm infants,” Shah said in a university news release.
The researchers used data from a study that has followed thousands of children since birth in 2001.
They tracked academic progress for 1,200 late preterm infants over time. They recorded developmental assessments at 9 months and 24 months, and followed up with reading and math scores at times when children would be in preschool and kindergarten.
While most late preemies developed reading skills on a schedule similar to their full-term peers, they had lower average math scores at all points. The biggest performance gap was in kindergarten.
It’s not clear why late preterm infants have vulnerability in math development but not in reading. Researchers said it could suggest unique brain development characteristics including structural changes in neural pathways related to visuoconstructive skills. Visuoconstructive abilities involve coordination of fine motor skills and tasks such as drawing.
“Now that we have identified patterns and predictors of reading and math skill development, we can help inform pediatric guidelines to help late preterm infants, who are the majority of infants born preterm, thrive in the period before kindergarten,” Shah said.
Study findings were published Aug. 25 in the journal Pediatric Research.

Golden Runs To His Baby Sister’s Crib Every Morning | The Dodo
Golden retriever is the first one to run to his baby sister’s crib every morning


Mental Health Services for Caregivers of Premature Infants
Yanique Williams-Adeniji, MSW, LICSW

Introduction: Globally, there are approximately 15 million preterm births annually (1), with almost 400,000 occurring in the U.S. The increase in preterm newborns continues to be a public health challenge. Preterm births, defined as birth before 37 weeks, continue to be the leading cause of morbidity and mortality. The focus of infant research is the reduction of preterm births, although the number of overall preterm births has not decreased. In 2021, the Centers for Disease Control and Prevention (CDC) reported that preterm births rose by 4%, with one in ten pregnancies classified as preterm . Moreover, when considering racial, ethnic, and social disparities, preterm births remain stagnant as Black Women account for 14.4% of all births, 50% higher than both White and Hispanic births. Twenty percent of pregnant women will experience some form of adverse mental health outcome while caring for their babies . Along with preterm birth outcomes, there is considerable concern for the mental health and wellness of pregnant persons. The physical health of the infant and pregnant person is often prioritized in healthcare settings at the expense of the pregnant person’s current and long-term mental well-being. With the current maternal mortality rate at 32.9 per 100,000 births and infant mortality at 5.4 per 1,000 per live births, it is not surprising that imminent health needs are paramount. Moreover, for minority populations, infant mortality remains the highest at 69.9 for Blacks and 28.0 for Hispanics. Although evidence supports health disparity in prematurity and mortality rates, both the physical and mental health concerns must be prioritized in maternal child health.
Mental Health Needs of Caregivers: Caregivers of premature newborns are more vulnerable to adverse mental health outcomes. These pregnant persons often suffer from anxiety, postpartum depression, post-traumatic stress disorder, and obsessive-compulsive disorder. If not assessed and targeted early in the perinatal period, the mother/infant bonding period is compromised. In addition, the cognitive development of newborns is compromised when pregnant persons suffer from mental/behavioral health challenges. Maternal mental/behavioral health challenges impact the entire family system. Maternal mental health challenges can pervasively impact the system, including the marital relationship, other children, and extended family.
Caregivers of premature infants have higher levels of stress and have unmet needs. In a study, pregnant persons reported that their mental health needs were not effectively addressed . Mental health services are identified as taking place during the antepartum period and consisting only of postpartum depression screens. There are opportunities to assess and screen significantly earlier in the perinatal period, allowing customized, comprehensive mental health treatment and services. Unique Needs of Women of Color: The preterm birth rate for Black women is 50% higher compared to both White and Hispanic pregnant persons . The premature birth rate for Black women is attributed to long-standing racial discrimination. Though the risk of mental health issues is high for all pregnant persons who give birth to premature newborns, studies have shown that women of color, particularly Black women, are at higher risk. Black women do not receive adequate mental health services that are culturally sensitive. Healthcare policies and practices must focus on cultural biases and racism. Black pregnant persons report feeling invisible and misunderstood by providers and hospital staff. The “one size fits all ‘’ health care model undercuts the multiple socio-cultural layers that affect Black pregnant persons. These types of experiences worsen mental health outcomes. More research is needed to explore the health care and mental health care needs of Black pregnant persons.
Reflections of a Mother: As a woman of color who gave birth to late preterm (35 weeks) twins during COVID-19, I can attest to the necessity of ongoing dialogue around mental health services for mothers. Though my children were fortunate not to have any major medical complications, both my genetics specialist and obstetrician used each perinatal appointment to prepare me for the possibility of a newborn intensive care unit (NICU) admission due to premature birth. Though I was given adequate information regarding the best and worst scenarios concerning the health outcomes of my twins, my anxiety increased with mood swings fluctuating from anxious to depressed with constant, ongoing hypervigilance. These mental health needs were not addressed. I was clear that being a woman of color placed me at higher, elevated health risks and adverse maternal health outcomes; however, I was not aware or prepared for the mental health toll during and after my pregnancy.
Interactions with healthcare providers were not ideal and further contributed to mental health challenges. Though I would voice my desire to carry my pregnancy to at least 35 weeks, I was told it was impossible due to my dynamic cervix. Early in my second trimester, I requested to be placed on bed rest; however, I was told I “was fine.” Since my husband was not permitted to attend my appointments due to COVID-19 health regulations, I requested that he attend virtually via Facetime. This request was met with resistance even though his support would have benefited my mental health. My husband would try to discuss my mental health challenges and voice his own concerns, but his concerns were also ignored. These instances often made me feel alone and that I had no autonomy over my pregnancy or my body. The lack of cultural sensitivity I encountered only heightened my fear and frustration. I, too, felt unheard, particularly when advocating for the mental health services I needed. Moreover, the lack of provider engagement after giving birth and at discharge left me anxious and concerned about my ability to effectively parent premature infant twins who were both under five pounds. Conclusion: The patient and health care provider relationship holds a vital key to shifting maternal mental health care services. Researchers propose using collaborative models when discussing mental health interventions to alleviate maternal stress (5). Furthermore, a host of perinatal mental health screening can detect other perinatal conditions outside of depression. Ongoing maternal health care assessments and interventions should not be limited to the hospital setting or discharge. Instead, the discharge plan should include follow-up reassessments and interventions to promote the continuity of care and progress.
As premature births continue to remain on the rise, adequate maternal mental health interventions are vital to the healthy development of newborns and serve an essential role in supporting pregnant persons during the perinatal period. When supporting the mental health of persons of color, health providers are tasked to create meaningful bonds for pregnant persons by listening, validating concerns and fears, and encouraging full family support. To help decrease stigma and increase mental health awareness, culturally relevant community education about perinatal mental health concerns and their impact on the perinatal postpartum.
Source:https://neonatologytoday.net/newsletters/nt-sep23.pdf

Premature Baby Alfie Arrives Three Months Early | Tiny Lives Series 2 | BBC Scotland
Jun 14, 2022

Born three months premature, Alfie and his family are having to take each day at a time. Tiny Lives Series two follows University Hospital Wishaw’s Neonatal Unit and their team of highly specialist staff who provide round-the-clock care to some of Scotland’s most fragile babies. This series follows the stories of babies born prematurely at the height of the Covid-19 pandemic in Scotland.


HEALTHCARE PARTNERS

Study identifies potential new way to protect premature babies from deadly infection
Reviewed by Danielle Ellis, B.Sc.Oct 4 2023
Premature babies in neonatal care units are extremely vulnerable, and susceptible to life-threatening infections. To help keep these babies safe the risk of infection needs to be kept as low as possible.
A particular problem is late-onset sepsis that starts from three days after birth, when bacteria get into the blood and grow. This can be very dangerous and babies with late-onset sepsis end up staying in hospital longer, need more treatment with antibiotics and can be left with life-long effects on their health.
Bacteria from the Staphylococcus family are the most common causes of late-onset sepsis. Most members of this large group of bacteria are harmless; they are normal colonizers of our skin, which can even protect us from harmful microbes. However, some strains, when they end up in the wrong place and get inside the body, can cause major problems, particularly for immunocompromised individuals like neonatal babies.
Staphylococcus capitis is an example of this. This is a species which is usually content living on our scalp, face and neck; capitis means “of the head’ in Latin. Some strains of S. capitis are however associated with late onset sepsis. One particular strain, known as NRCS-A, has been identified as causing serious infections in neonates around the world.
Scientists think this strain first emerged in the 1960s and spread globally throughout the 1980s as it evolved resistance to the commonly used antibiotic vancomycin. Strains circulating now show resistance to multiple antibiotics and a reduced susceptibility to antiseptics that we use to sterilize the skin of babies. This makes the bacteria harder to treat and control, but exactly why this NRCS-A strain has become so globally successful has remained a mystery.
To try and understand what makes this strain able to spread around the world and to develop better ways to keep it under control, Professor Mark Webber and his team from the Quadram Institute and University of East Anglia analyzed the genomes of hundreds of S. capitis isolates. They worked with two Neonatal Intensive Care Units (NICUs), one in the UK and one in Germany, obtaining samples of S. capitis from the skin and gut of neonatal babies, with and without late onset sepsis.
Their results, published in the journal Microbial Genomics, found that the NRCS-A strain was commonly carried on the skin and in the gut of uninfected neonatal babies, that transmission between babies within NICUs was likely.
By reading the complete genome of each sample, the team were able to identify tiny genetic differences between the S. capitis strains that caused disease and those that don’t.
Professor Webber and his team found that the NRCS-A strains that can cause disease carried a set of unique genes, which they think allows them to survive in the gut as well as on the skin. This would make cleaning the skin to eradicate the bacteria ineffective as the babies will carry a reservoir in their gut microbiomes that cannot be easily removed, but can act as a source of infection.
The genes found in the NRCS-A strains allow them to be resistant to nisin, an antimicrobial compound naturally produced by bacteria in the gut. They also carried genes to survive exposure to the toxic metals that our immune system uses to kill bacteria, as well as genes to scavenge essential metals that are known to be hard for bacteria to access in the gut environment.
Further experiments also showed that the bacteria grow better in acidic conditions as found in the gut. Together, the evidence supports the idea these bacteria are adapted to exploit growth in the gut.
If metal scavenging is critical to infection, this may also be the bacteria’s Achilles heel, presenting a new way to counter its threat. There is early evidence that feeding babies a probiotic supplement of benign bacteria reduces the rate of late onset sepsis and that these ‘good bacteria’ can extract metals before the S. capitis, preventing their growth.
“Studying how strains like NRCS-A have become globally successful is crucial to understanding how bacteria evolve to colonize different environments, and to give us new ideas about how to reduce the risks of infection in vulnerable populations” said Professor Webber.
“We hope this work can be the starting point for more research to develop better ways to protect newborn babies from the terrible consequences of infection.”
Source: Microbiology Society
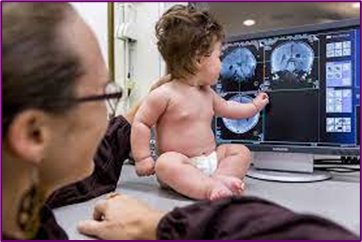
Zooming in on the brains of babies
New tools are helping neuroscientists investigate why early life is such a crucial time for neural development
By Emily Underwood 03.20.2023

Many of our defining traits — including the languages we speak and how we connect with others — can be traced back at least in part to our earliest experiences. Although our brains remain malleable throughout our lives, most neuroscientists agree that the changes that occur in the womb and in the first few years of life are among the most consequential, with an outsize effect on our risk of developmental and psychiatric conditions.
How maternal mood shapes the developing brain
“Early on in life, the brain is still forming itself,” says Claudia Lugo-Candelas, a clinical psychologist at Columbia University and coauthor of an overview of the prenatal origins of psychiatric illness in the Annual Review of Clinical Psychology. Starting from a tiny cluster of stem cells, the brain develops into a complex organ of roughly 100 billion neurons and trillions of connections in just nine months. Compared to the more subtle brain changes that occur later in life, Lugo-Candelas says, what happens in utero and shortly after birth “is like building the house, versus finishing the deck.”
But just how this process unfolds, and why it sometimes goes awry, has been a hard mystery to crack, largely because so many of the key events are difficult to observe. The first magnetic resonance imaging (MRI) scans of baby and fetal brains were taken back in the early 1980s, and doctors seized on the tool to diagnose major malformations in brain structure. But neuroimaging tools that can capture the baby brain’s inner workings in detail and spy on fetal brain activity in pregnant moms are much newer developments. Today, this research, coupled with long-term studies that follow thousands of individual children for years, is giving scientists new insights into how the brain develops.
These advances have propelled researchers to a different stage than they were in even five years ago, says Damien Fair, a neuroscientist at the University of Minnesota who studies developmental conditions like autism and attention deficit hyperactivity disorder (ADHD).
Until recently, a major challenge has been that, unlike an adult, a fetus or newborn baby won’t lie still inside a brain scanner. Buoyed by amniotic fluid, a fetus constantly shifts position, and newborn babies love to wriggle around, checking out their environment. In the past, researchers and clinicians often had to do multiple time-consuming, expensive scans to get a good image. They sometimes sedated children and pregnant moms to reduce movement, an approach that alters brain function and may have health risks.
But new imaging and computational techniques that reduce distortions caused by fidgeting — including software developed by a company cofounded by Fair — have made it easier to collect data from babies and fetuses. And that has invigorated the field.
Peering into prenatal brain development
The new work is starting to reveal what typical brain development looks like and hint at how atypical conditions like autism and ADHD may arise. In a first-of-its kind study in 2017, for example, a team of researchers led by pediatric neuroscientist Moriah Thomason, now at New York University, used functional magnetic resonance imaging (fMRI) to investigate patterns of neural communication among brain regions in 32 fetuses. Half of the pregnant women were at high risk of early delivery and 14 of the babies ultimately were born prematurely.
Premature birth is a known risk factor for cognitive and emotional issues later on. But it has been difficult for scientists to determine whether this is due to the trauma of premature delivery, which often involves brain injury and oxygen deprivation, or to preexisting brain differences that start in the womb.
Thomason’s study provided the first evidence that the problems start in utero.
As fetuses, the preemies-to-be that were scanned by her team had brain activity that suggested weaker communication between various brain regions compared with fetuses that ended up being carried to term. Most strikingly, the scientists found altered neural communication in networks that eventually support language, including a language center on the left side of the brain.
Researchers have since found more evidence for prenatal brain disruption in preemies. In 2021, for example, another group found that 24 prematurely delivered infants had lower brain volumes and less cerebrospinal fluid while still in utero, compared with a group of infants carried to term. And a variety of studies have found that women who delivered prematurely had high levels of inflammation caused by bacterial or viral infections in the amniotic fluid and placental tissues.
The findings add to growing evidence that inflammatory events during pregnancy can alter fetal brain development. Large-population studies, for example, have shown that mothers who have had a severe infection during pregnancy are at a slightly elevated risk of having an autistic child, although it’s not yet clear that prenatal infection alone can actually cause autism.
Lugo-Candelas’s research focuses on how a pregnant woman’s perceived stress, life events, depression and anxiety may affect early brain development. A number of studies have found that high maternal anxiety and depression during pregnancy are associated with a twofold increase in the risk of the child developing a mental disorder later in life. If the risks start earlier in development, “that also means there’s a chance to intervene earlier than we thought,” she says. But, Lugo-Candelas adds, scientists are still working to untangle the mechanisms behind that increased risk, what stressors might have the most impact, and when and how to intervene.
An MRI scan shows MIT neuroscientist Rebecca Saxe kissing her 2-month-old son. Advances in imaging software have allowed researchers to better study the changing brains of babies.
Moreover, like many other risk factors in pregnancy, there’s no one thing that leads to psychiatric illness or developmental problems, says Lugo-Candelas. “It’s a collection of tiny risks.” She emphasizes that there’s nothing rigidly deterministic about any of these early exposures or experiences. “You can have children that are exposed prenatally to a bunch of the things that we think could increase risk for a psychiatric disease, and then have a child that doesn’t have a disorder at all and will never have it.”
That complexity speaks to one of the greatest challenges of studying the developing brain: the fact that similar outcomes, such as autism or schizophrenia, can have many underlying neurological causes. Some people with autism have increased connectivity between certain brain regions compared with the neurotypical population, for example — but others have less. There’s no single neural signature for the condition.
Brain connections as ‘neural fingerprints’
Fair’s approach to this problem has been to identify what he calls “functional fingerprints,” patterns — unique to each individual — in how different brain regions communicate with each other when a person is at rest inside an fMRI scanner.
He first observed these neural fingerprints in adults in 2014, and went on to show that children have them too. The patterns are surprisingly consistent within families, even across generations, he and his colleagues have found, suggesting that certain types of brain connectivity are at least partially inherited.
Neuroscientists at MIT have made their brain imaging set-up more baby-friendly to learn more about early development. Using an adapted MRI scanner, researchers can image infants’ brains as the babies watch movies with different types of visual stimuli.
Last year, he published evidence that even eight-month-old babies have these neural fingerprints — and that certain elements of the fingerprint, such as the amount of crosstalk between regions involved in functions like attention and movement, can predict an infant’s precise age, down to a few months.
Meanwhile, Thomason’s fMRI studies of the fetal brain suggest that these distinct connectivity patterns emerge in the second and third trimester, including in neural circuits that eventually govern learning, memory and emotion. Thomason and others are now using neuroimaging to investigate how a variety of prenatal experiences — ranging from maternal Covid-19 infection to cannabis use — affect how these circuits develop.
The fact that scientists can detect these distinct brain activity patterns so early suggests to Fair and others that much of what makes us who we are is already in place by the time we’re born, even though we’ll continue to be shaped by our experiences and exposures throughout life. Because every baby’s brain is shaped by so many different factors, however, researchers are going to need long-term imaging data from thousands of children to get a robust understanding of what “typical” development looks like, Fair and colleagues argue in the 2021 Annual Review of Developmental Psychology.
Eventually, imaging tools could help clinicians and researchers monitor how a baby’s brain is developing, spot signs of future trouble and develop earlier personalized interventions and treatments for conditions like autism, Fair adds.
In the meantime, Lugo-Candelas thinks that we already know enough to take action. “I feel pretty confident that interventions that effectively minimize distress in pregnancy, like paid maternal leave, are going to be beneficial for the next generation,” she says. She notes that could lead to better outcomes in school and other areas, like mental health, that ripple across the lifespan. “I just don’t think we’ve done a really good job yet at measuring what those outcomes look like, or the mechanisms that lead to them.”
Source:https://knowablemagazine.org/article/mind/2023/zooming-brains-babies
A Call for Change: Fixing A Broken Medical Training System | Jake Goodman |

15,359 views May 22, 2023
Medical training practices in the United States haven’t changed much since formal residency programs were first introduced in 1897. A series of unaddressed problems within these practices have perpetuated mental health challenges within the medical profession. In this talk, Dr. Jake Goodman brings awareness and promotes advocacy to further the discussion on medical training improvements that are necessary to better protect the mental health and care of both physicians and patients. Jake Goodman is a Miami-based psychiatry resident physician. With more than 2.1 million followers, Dr. Goodman is a mental health activist and social media content creator focused on fighting stigma and discrimination while empowering those experiencing mental health challenges to seek help. This talk was given at a TEDx event using the TED conference format but independently organized by a local community.



“Adventure Awaits: Facing Obstacles with a Smile!”
Hey there, brave adventurers of all ages! Life is like a grand treasure hunt, full of twists, turns, and… obstacles! But guess what? These obstacles are like secret doors to even more exciting adventures! 🌟
Imagine you’re climbing a giant mountain, and suddenly, you come across a massive boulder blocking your path. Instead of giving up, let’s put on our explorer hats and get creative! Maybe you can find a clever way to go around it or even turn it into a rock-solid stepping stone to reach new heights! Remember, it’s not about the size of the obstacle; it’s about the size of your openness and receptivity to new pathways! Obstacles can teach us incredible things, like patience, creativity, and solution generation. So, next time you find yourself face-to-face with one of life’s challenges, remember this: you’re an intrepid explorer on a grand adventure! Don’t forget to share your stories ofobstacle-conquering with your fellow adventurers and make every moment a fantastic part of your journey. Embrace the bumps, twists, and turns with a smile because the best is yet to come!! 🚀💫
Resilience In Hard Times
At the very darkest points of individual and national life, we need – more than ever – to practice the art of resilience. If you like our films, take a look at our shop (we ship worldwide):

Turning adversity into opportunity | Muniba Mazari | TEDxIslamabad
In this talk Muniba shares the heart wrenching story of an incident which changed her life completely – from the bad to the good. Muniba Mazari is an artist and a writer. She believes in playing with vibrant colors and flawless portrayal of true emotions. Her work speaks her heart out and is all about people, their expressions, dreams and aspirations.
Although wheel chair bound, her spirit and artistry knows no bounds. In fact, she takes the agony of spinal cord injury as a challenge and is more determined to express her sentiments through her art work. While doing her bachelor in fine arts she met a road accident which made her paraplegic. Currently, she is running her brand by the name ‘Muniba’s Canvas’ with the slogan ‘Let Your Walls Wear Colors’. She is a mix media artist and believes in depicting the ethnic jewels of her region in an abstract way. Some of her work is purely abstract which depicts the humans’ expressions, their thoughts and dreams. Her paintings give the message of living life and represent the real personality of the artist.



Book: My Early Surprise: A Bedtime Story For Preemies Author: Sharifa Brown

Join me as we read, “My Early Surprise: A Bedtime Story For Preemies” by Sharifa Brown. Here we see first-hand the obstacles Baby Malik and his family faced during his early entrance into the world!

No Surfing in Mongolia but Amazing Skiing

Altain Tavan bogd Snowboard Mongolia Ski Mongolia

Alex Tino Jan 24, 2020 #ski #snowboard #mountain